AIVIS to Showcase ‘Qanti IHC’ AI-Powered Diagnostic Software at KIMES 2025
AVING
February 12, 2025
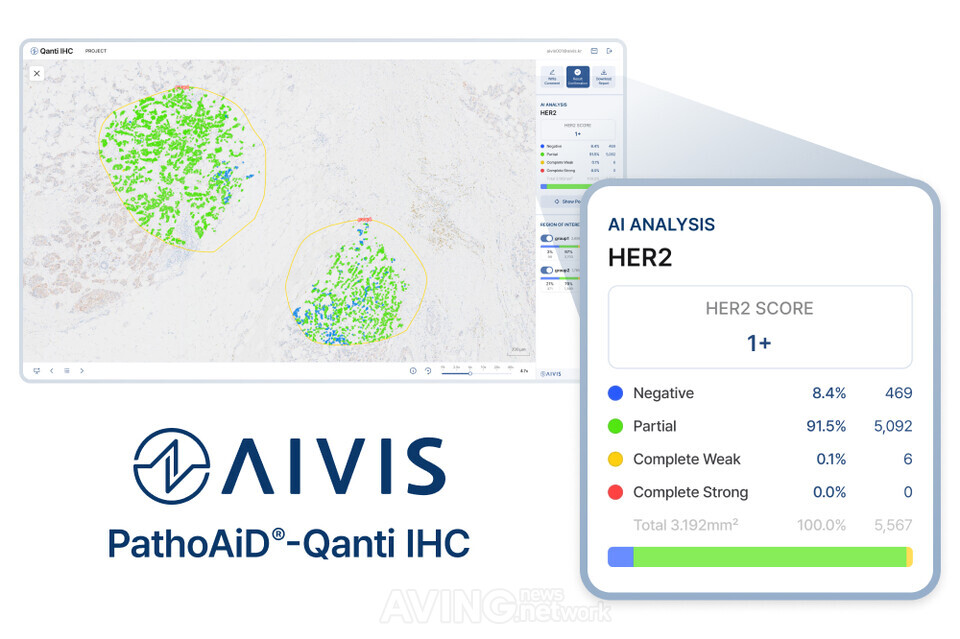
AIVIS Inc. (CEO Lee Daehong) announced that it will participate in KIMES 2025—the 40th International Medical Device and Hospital Equipment Exhibition—at COEX from March 20 (Thursday) to March 23 (Sunday), showcasing its innovations at the “KIMES INSPIRE” special pavilion. AIVIS was founded by a team of AI researchers with Master’s and Doctorate degrees in image processing and artificial intelligence from Korea University, in collaboration with physicians. The company’s mission is to provide digital pathology solutions that enhance the precision of cancer diagnosis and treatment. Established in 2021, AIVIS developed a proprietary algorithm for analyzing pathology images using artificial intelligence, and it has secured an innovative biomarker analysis technology that quantifies even HER2 ultra-low expression. Its technological excellence and safety have been validated through regulatory approval from the Ministry of Food and Drug Safety, as well as ISO13485 and GMP certifications. Collaborations with global industry leaders such as Philips and AstraZeneca have further confirmed its product compatibility and scalability. Currently, AIVIS is developing and supplying AI diagnostic support solutions for various cancers, including breast, thyroid, and colorectal cancers, and has introduced its digital pathology-based AI products in numerous medical institutions both domestically and internationally. The company is continuously advancing research and market expansion efforts to establish itself as a global leader in digital pathology. Domestically, AIVIS has completed proof-of-concept (PoC) verifications at over 20 major medical institutions—including Seoul St. Mary’s Hospital and Sinchon Severance Hospital—providing quantitative analysis services for key biomarkers such as HER2, ER, PR, and Ki-67, along with AI-based cancer diagnostic support. Notably, the company has entered into a licensing agreement with Philips for its biomarker quantification solution, achieving enhanced workflow efficiency in pathology departments through integration with digital pathology scanners. Internationally, AIVIS is preparing for market entry by collaborating with hospitals in the ASEAN region. In addition, a joint promotion with global pharmaceutical giant AstraZeneca is scheduled to screen patient groups with HER2 Low/Ultralow expression. AIVIS is also striving to enter the companion diagnostics (CDx) market for new drug clinical trials by leveraging its AI pathology analysis service. The company is in the process of obtaining regulatory approvals in APAC markets such as Australia, with the aim of increasing its overseas sales through proactive local partnerships. Building on its domestic and international success stories, AIVIS plans to expand its lineup of biomarker quantification products and extend its analytical services to various cancer types. At the exhibition, AIVIS will present its flagship product, “Qanti IHC (PathoAiD-Qanti IHC),” a software medical device approved by the Ministry of Food and Drug Safety. This device automatically quantifies key biomarkers—such as HER2, ER, PR, and Ki-67—to enhance diagnostic accuracy for pathologists. With its proprietary AI algorithm capable of detecting even HER2 Low/Ultralow expressions, the solution enables objective and precise diagnoses in areas that have been challenging to discern through conventional pathology. The solution is distinguished by its ability to rapidly process ultra-high-resolution images extracted from digital scanners, and its patent-backed technology efficiently analyzes large-scale pathology data. Moreover, it easily integrates with Philips scanners and pathology viewers, allowing the seamless adoption of AI analysis functions across various environments, including cloud and on-premises setups, while maintaining existing hospital infrastructure. Finally, its user interface and experience—designed through close collaboration between pathologists and engineers—maximize on-site usability and ensure smooth integration into the pathology diagnostic process. AIVIS stated, “Through this exhibition, we aim to showcase the practical value of our AI pathology solution in establishing precise diagnostic and treatment strategies for hospitals, pharmaceutical companies, and medical device manufacturers worldwide. Additionally, we plan to secure more global networks by engaging with global medical device scanner companies and participants from pathology professional associations, expanding our collaborations beyond our current partners such as Philips.” They added, “By building these networks and showcasing the technological capabilities of our product lineup—including our HER2 ultra-low quantification solution and our offerings for thyroid and colorectal cancers—we seek to foster joint research and commercialization collaborations in new drug development and companion diagnostics. Our goal is also to create substantial opportunities for overseas sales and investment attraction by appealing to international buyers and investors through our proven success in domestic medical institutions.” AIVIS has prioritized Australia, Indonesia, and India as its initial targets in the APAC region—where digital pathology infrastructure is rapidly expanding—and plans to actively expand its partnerships with Philips APAC and Roche Innovation. Through these efforts, the company aspires to establish its AI pathology solution throughout the APAC region and strengthen collaborations with global medical device and pharmaceutical partners, ultimately securing a leading position in the digital pathology market. KIMES, which began in 1980 and has grown alongside the development of Korea’s medical industry, is the country’s largest exhibition specializing in medical devices and hospital equipment. KIMES 2025, co-hosted by Korea I&X, the Korea Medical Device Association, and the Korea Medical Device Industry Association, will feature cutting-edge medical devices and hospital equipment from around the world. Furthermore, the exhibition will feature a “KIMES INSPIRE” special pavilion, which will gather approximately 20 innovative companies from the fields of medical startups, SaMD (software as a medical device), and digital healthcare to showcase the vision for future medical technologies. This event is set to highlight the advancement of digital healthcare and AI software through innovative technologies and creative solutions, outlining the emerging trends in the medical industry.